Nov 16, 2020 | Events, News
i-CONSENT organizes a series of workshops to discuss informed consent for clinical trials in the context of an emergency setting i-CONSENT partner, LUMSA University, has organized a series of internal online workshops to discuss informed consent in the...

Nov 12, 2020 | News, Publications
Project publishes an article describing informed consent in biomedical research in the pandemic context in the Journal of BioLaw: Rivista di Biodiritto Clinical research is world widely speeding in order to identify as soon as possible COVID-19 treatment and vaccine....
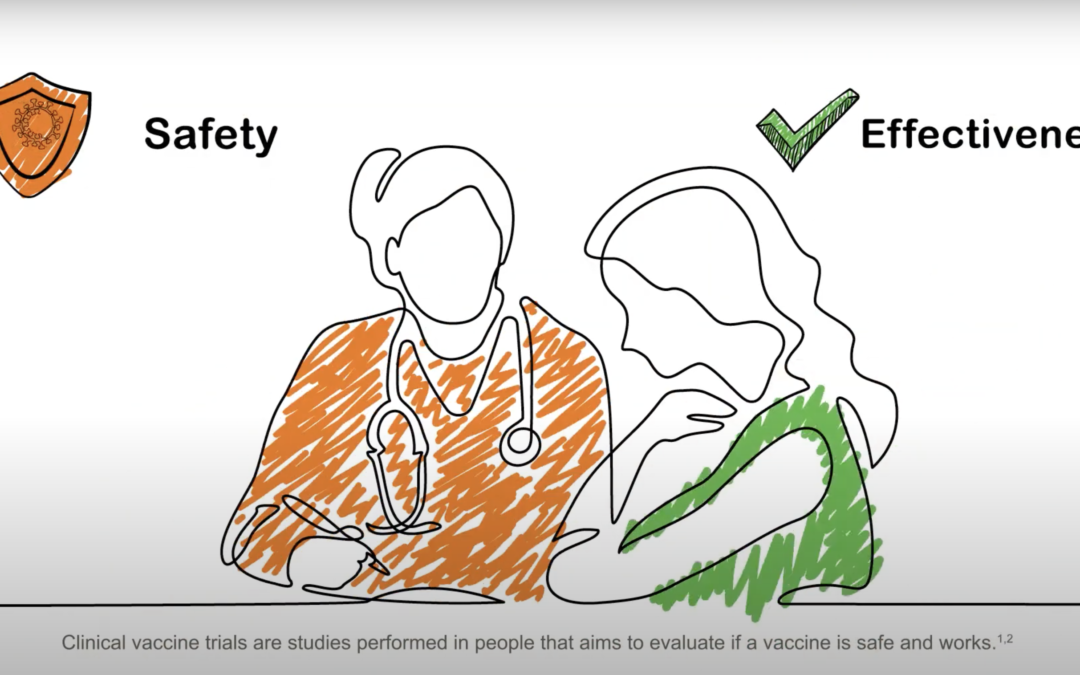
Oct 14, 2020 | News, Press Release
The video describes the development of clinical vaccine trials and explains all you need to know before choosing to participate in one The video, elaborated by project partner GSK, explains vaccines’ lifecycle from its production in the laboratory to its...