LUMSA University meets with cultural mediators in Rome to test Design Thinking
LUMSA University has organizanised in conjunction with OPBG a series of encounters with cultural mediators to test a new design methodology: Design Thinking.
Last Wednesday was the kick-off to the activities, divided on 3 days (Presentation, Workshop 1 and Workshop 2). During the briefing, Mara Zampol, Executive Assistant at OPGB‘s Epidemiology Department, was in charge of explaining the methodology that will be studied and applied in the following workshops. Design Thinking is a design methodology that provides a solution-based approach to solving problems. This methodology emphasizes:
The importance of teamwork;
The importance of exposing ideas in a peer-to-peer way;
The importance of generating ideas from the ideas of others (this allows you to grow and expand your world).
The Design Thinking provides five stages.
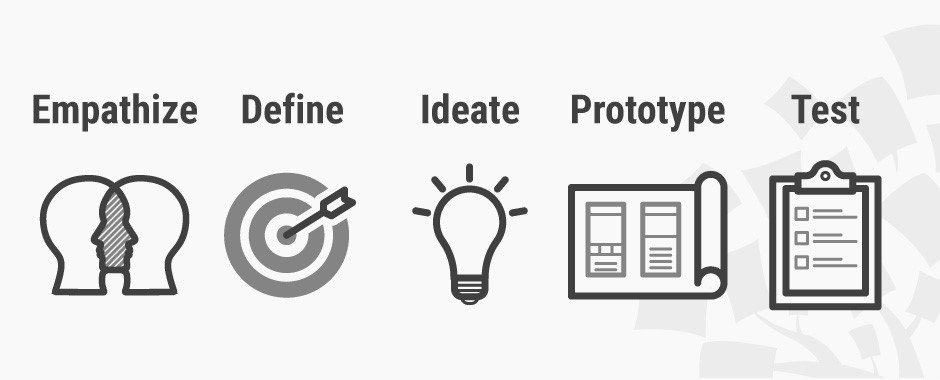
The conferences are framed within i-CONSENT Task 2.6 – New strategies for the inclusion of people coming from different cultural and religious backgrounds in clinical trials of the project.
After the presentation participants opened a brief discussion on some relevant aspects related to informed consent.
- One of the mediators referred to the case of an Afghan woman who decided to sign informed consent only after looking to her husband/following his opinion. This example shows the importance of reflecting about autonomy in the case of different cultures.
- On the ethical and legal side, researchers should look for an autonomous decision to take part in clinical research. In the case above, legally we have her signature but maybe she does not participate voluntarily. i-CONSENT should improve guidelines in the sense of promoting autonomy and also an increased access to information in the consent process.
- Trust is an important element in the consent process and it becomes even more significant when we deal with people coming from different cultural and religious backgrounds; also having enough time to read the consent form, to ask questions, is an important aspect. In the context (structure) of informed consent is equally worthy of attention.
- When the cultural mediator steps into the researcher-participant relationship, he/she has a direct relationship with the patient (or research participant). Perhaps even talking about the empathy of cultural mediators, rather than just the empathy of the doctor, would be more appropriate.
- The understanding of information in healthcare and in clinical trials is crucial because the subject is deciding about her/his body.
- Cultural mediation is different from linguistic mediation and from translation. Cultural mediation in healthcare and in clinical research needs the knowledge of specific medical language.
Next encounters, on February 7th and 28th, 2019 will apply the method studied during the meeting specifically on HPV, which will help to focus the scope on gender and minors. The conclusions of these conferences will be of great value to the project’s final results.





