i-CONSENT has elaborated examples of communication materials to be used in clinical trials following the project recommendations. The idea was to elaborate a series of materials to deliver all the necessary information to potential participants based on the project recommendations.
The materials have been create for three hypothetical clinical trials with vaccines: HPV in adolescents, RSV in pregnant woman and meningococcal disease in healthy adults. A part from accounting these 3 different settings, the materials take into consideration differences in the way of communication and patterns of reading. Also, interests and preferences in the use of media channels and/or new technologies.
To involve the potential participant from the beginning of the informed consent process, reflecting their specific needs and the before-mentioned features in different population groups, the team used patient-centred techniques. Within other, Design Thinking sessions and online surveys where conducted. Also, the materials were elaborated in different languages to test their implementation in different countries.
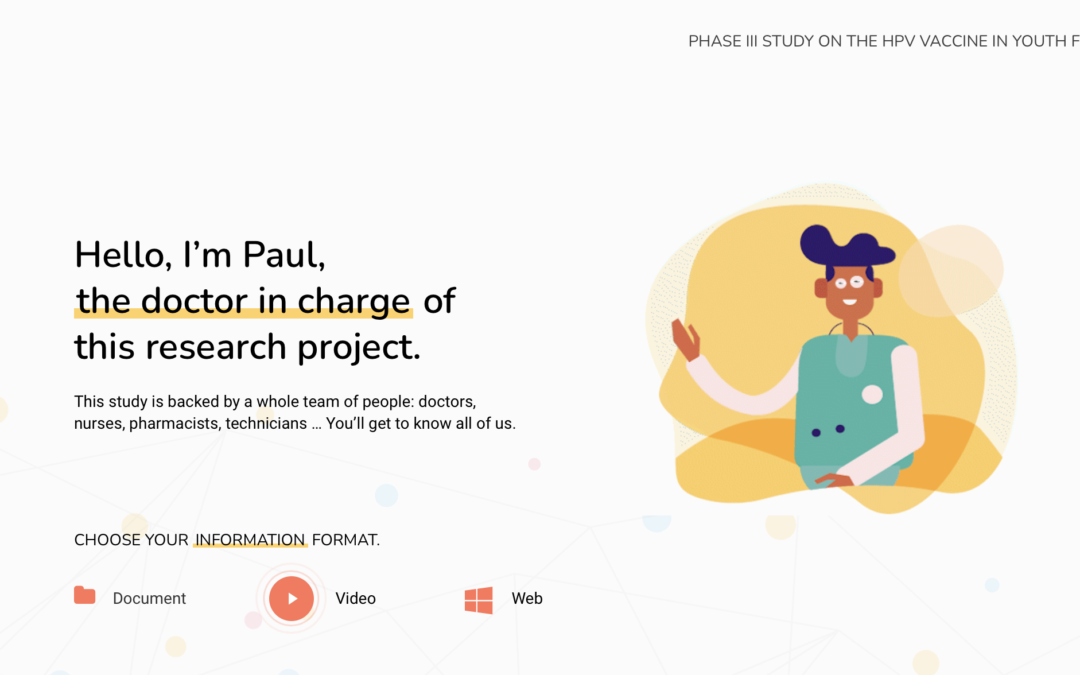
Human Papiloma Virus (VPH) in adolescents
The first case study is based on a clinical trial with a vaccine against the HP and involving healthy adolescents (females and males) 12-13 years of age. For this target group, a website with all the relevant information was created.

Information was also included on a cartoon video:
The information is also available on a plain written document, for those who might prefer this alternative:
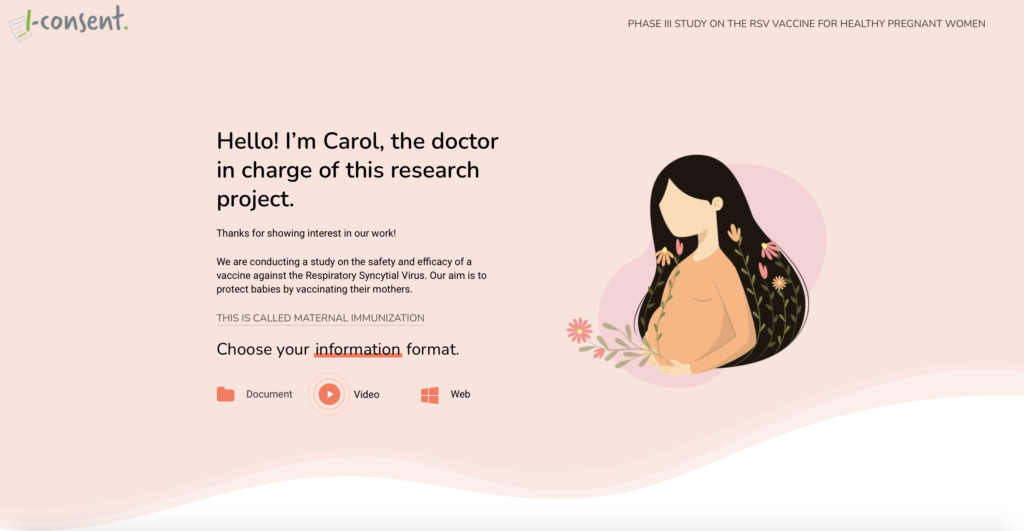
Respiratory Syncytial Virus (RSV) in pregnant women
The second case study is based on a clinical trial of a vaccine against the RSV infection in healthy pregnant women. Same formats as for adolescents where elaborated for this targets. Although adapted to their preferences and characteristics. Check the website here, including the written document and the video.
Meningococcal disease in adults
The third case study is based on a clinical trial of a vaccine against meningococcal disease in healthy adults. These where the materials elaborated for them:
The materials are currently being validated in the different settings.