The first i-CONSENT informed consent is now circulating. A real Spanish clinical trial has used the project guidelines to elaborate a series of communication materials to provide the information on the study to the participants. The ethics committee has not only approved the materials as supplementary information content but also as an informed consent form and participant information sheet. The materials consist of a webpage, a video and a informed consent document.
This innovative study, which explores the possibilities of decentralized clinical trials, has upgraded a basic informed consent model taking into account the recommendations of the i-CONSENT guidelines. Then, the team has translated the document into a webpage, where the information is distributed in layers according to the level of relevance. This level of relevance was agreed with a group of stakeholders during a series of interviews.
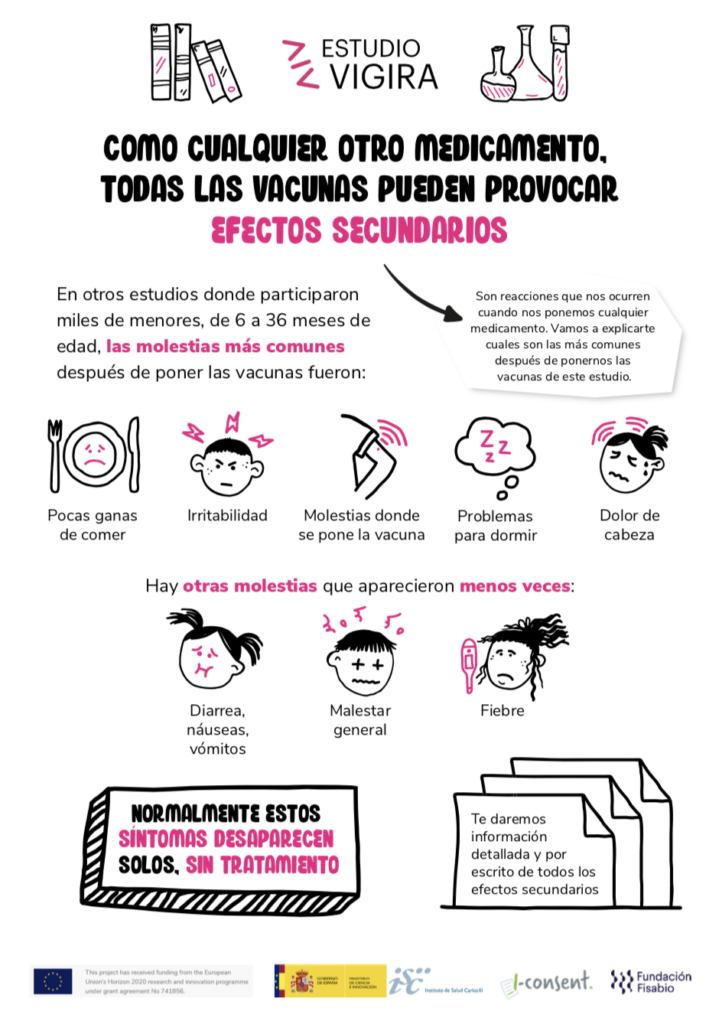
In addition, the webpage, which includes a video with an overview of what the participation in the trial will entail, includes a series of infographics with relevant concepts.
The study, called VIGIRA, is a Spanish Health Research Fund (FIS) funded project designed by the Carlos III Health Institute (ISCIII). The goal is estimating the effectiveness on influenza vaccines against influenza and other acute respiratory infections in children aged 12 to 35 months. The clinical trial is coordinated by the i-CONSENT Coordinator partner FISABIO.
