This virtual workshop organised by i-CONSENT partner AND-CG offered the opportunity to explore different dimensions of informed consent-related practices
Around 15 experts gathered yesterday morning for the i-CONSENT webinar «Rethinking informed consent: Emerging challenges in the digital era». The discussion revolved around the idea of informed consent (IC) from different perspectives, more specifically new technologies and the current regulation landscape.
The workshop was organised in two sessions. The first one was dedicated to future perspectives regarding IC including secondary uses of clinical trial data and consent solutions for this purposes and implications of Big Data for informed consent processes.
The second session focused on regulations applied to informed consent. Attendees could hear a representative of the Bioethics UNIT of the Council of Europe who explained their work in this regard. The last intervention of the afternoon explored the idea of GDPR in clinical trials under the supervision of the European Data Protection Board.
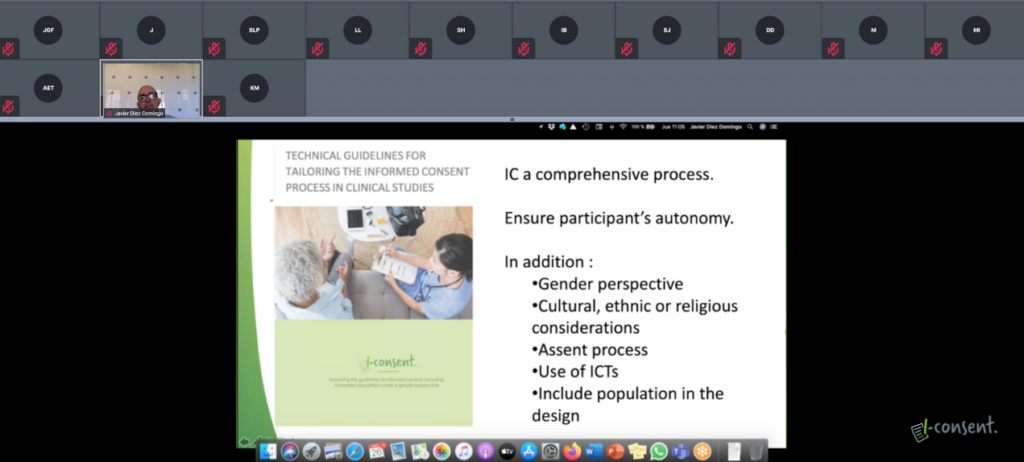
The discussion was lead by experts from around Europe including representatives of Acontrario Law, a business law firm specialized in IP, IT and data protection law, ETH Zurich, a university for science and technology, the University of Manchester or, as mentioned, the Bioethics Unit of the Council of Europe.
Very interesting discussions arose, and a report will be soon available on our website.