The project has made a selection of the most frequently asked questions in internet related to informed consent for Health Literacy Month
October is Health Literacy Month. To celebrate it, i-CONSENT has decided to explore people’s questions regarding informed consent as closely as possible. One of i-CONSENT’s main objectives is to bring science closer to the wider public by unlocking health knowledge for their use. For this reason, we have answered a selection of the most frequently asked questions related to informed consent.
What does informed consent mean and why is it important?
Informed Consent is a key aspect in the decision process to participate in a study. During the Informed Consent process participants learn the most relevant aspects of the study and formally state if they accept to participate in it or not.
i-CONSENT defines the informed consent process as “a bidirectional communication process that begins with the first contact with the potential participant and continues throughout the study until its end. During this process, continuous feedback and communication between the participant and the research team are essential.”
COVID-19 has brought changes in the procedures followed by clinical trials, in order to decrease the risk of contagion by participants. The investigators should inform trial participants, in a timely manner, about changes in the conduct of the clinical trial relevant to them.
When was informed consent established?
The idea of informed consent had its origin in the political philosophy of the 16th century. Experts defended that human relationships couldn’t be based on vertical relationships. Even so, its use has become normalized in medicine during the 20th century. The first consent documents date back to the 16th century, in the old Ottoman Empire, where contracts were signed before performing surgical interventions.
Although its introduction into the judicial lexicon was in 1957, during a case in the medical court, the term “informed consent” appears named as such for the first time in a medical research ethics document in the first revision of the Declaration of Helsinki (1975).
When is informed consent required?
The informed consent process should begin before you start any treatment, surgery, clinical trial or study involving your health. When talking about informed consent i-CONSENT Project, focuses specifically on informed consent in clinical studies.
A clinical study involves research using human volunteers (also called participants) and is intended to add to medical knowledge. Informed consent in this case will confirm that the participant understands the objectives of the study, what his/her participation entails, risks and benefits, etc. And that accepts to participate.
The participant may be required to re-consent if any modification in the study occurs. This ensures that he/she still understands the study and is still willing to continue in it.
COVID-19 is a reality now present in clinical trials. European agencies have drawn up a series of guides in which some specifications on how to deal with COVID-19 are included. Here you can find the EMA’s Guidance on the management of Clinical Trials during the COVID-19 (Coronavirus) pandemic.
How is informed consent an ethical dilemma?
One of the main ethical challenges in the informed consent processes is the wrong idea that it is a detailed technical and exhaustive description of a clinical study. In the approach, its exclusive aim is defend the investigators. The truth is that it should be designed to protect the subjects or potential subjects. The informed consent is a process where the patient is taught about his/her health and is empowered to make autonomous decisions.
Some other emerging ethical issues at stake are given by dynamic informed consent; informed consent process personalization; technological innovation, including the use of social media during the recruitment of participants; comprehension verification; physician’s training in communication; health literacy for participants within others.
Of course, an additional challenge nowadays is posed by COVID-19. The priority should always be the participants’ wellbeing. For example, research team and participant should reduce face to face interactions without compromising the informed consent process.
Can informed consent be verbal?
Redes Sociales: Yes, informed consent can be verbal. Modern technologies allow for remote interventions and interaction between doctors and patients through digital media. For instance, recording a video with one’s voice can be as good as signing a paper. Even so, in most of the cases, verbal consent at the time of trial enrolment has be followed, at a later stage, by written consent.
During COVID-19 pandemic alternative ways of obtaining consent and re-consent are being considered, including oral consent via phone or video-calls. This should always be documented in the trial participants’ medical records and supplemented with e-mail confirmation.
Can informed consent be withdrawn?
The participant can withdraw their consent anytime throughout the study. There is no need to give explanations and it will have no consequences for the participant’s health.
How will informed consent be in the future?
In the future, we will rely more and more on new forms of informed consent. eConsent, the electronic version of informed consent, is a reality already in use and it will only increase.
eConsent provides an interactive experience for participants that aligns with their unique preferences, needs, and learning styles. The solutions given by this alternative have the potential to help provide a tailored experience to patients, enhance enrolment and retention rates. Ultimately empower participants to make more informed, knowledgeable decisions about their care.
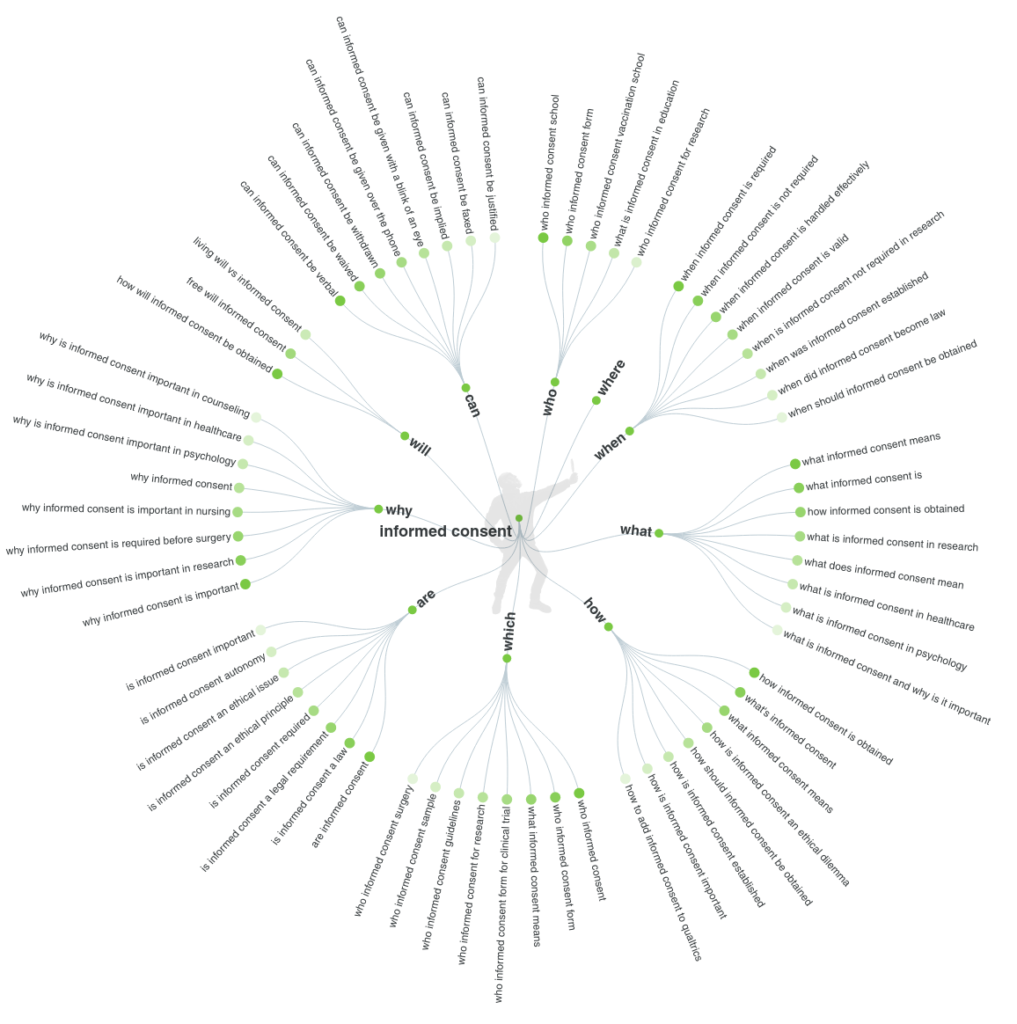
The methodology used to select the questions has been the online app Answer the Public. This tool processes data from search engines like Google and extracts every useful phrase and question people are asking online around the selected keyword. The questions where a result of a search in the 3 project consortium languages (English, Spanish and Italian), and a merge of the main common doubts.
Contact the team with any additional questions around informed consent that you come up with. We will be happy to discuss them with you!