i-CONSENT: A new perspective
Informed Consent is a key aspect in the decision process to participate in a study. During the Informed Consent process participants learn the most relevant aspects of the study and accept to participate in it or not. At present, most of Informed Consent documents are complex, difficult to understand and drafted without patients’ perspective. There is a need to improve informed consent process through the implementation of innovative proposals adapted to participants needs. This new perspective offered by the i-CONSENT project will empower the participant to voluntary decide to participate in the study and facilitate and improve its autonomy in decision making and increase his/her satisfaction.
Objectives
The i-CONSENT project has 6 main objectives
1. Assess all stakeholders’ needs in relation to Informed Consent.
2. Identify gaps, barriers and challenges in the process of Informed Consent.
3. Analyse risks and opportunities of using digital technologies (ICT).
4. Develop tailored strategies for the development of Informed Consent.
5. Analyse the legal aspects of Informed Consent in relation to Social Media use.
Outcomes
Workshops
Interaction with stakeholders to assess their needs
Innovative Resources
Develop new resources to facilitate the understanding of Informed Consent using digital and non-digital tools
Guidelines
Develop, validate and publish comprehensive guidelines to improve the Informed Consent process
Publications
Production of scientific publications and contents for professionals and general public.
Fundamentals of Informed Consent Process
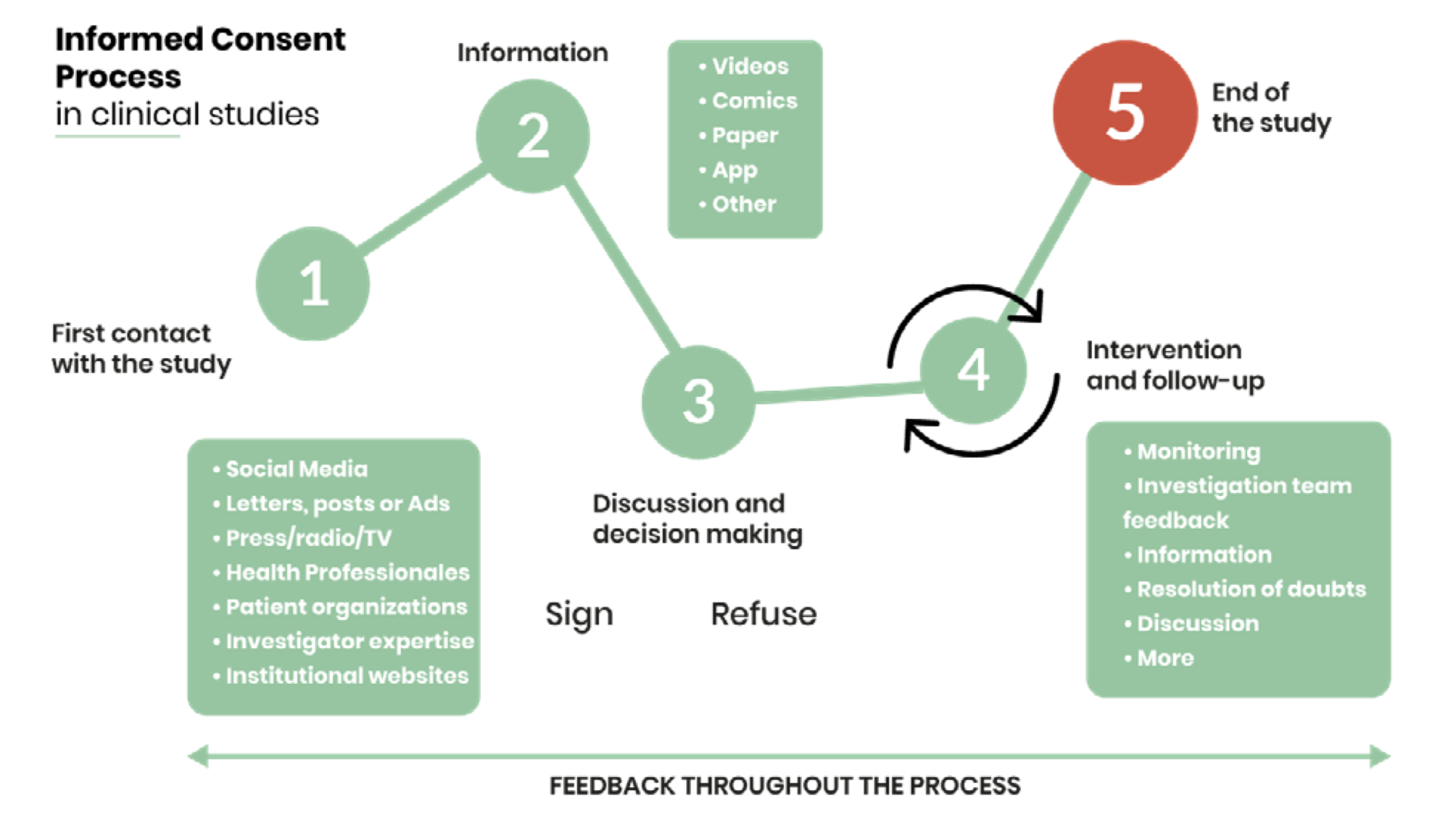
i-CONSENT understands informed consent as a bidirectional communication process that begins with the first contact with the potential participant and continues throughout the entire study until its end.
Once the potential participant has shown an interest in the study, the investigation team will provide further information through different channels (videos, comic, app, website…). After processing the content, the potential participant meet the investigator to resolve any doubts about the study and his/her participation to ensure that has enough knowledge and information to make an autonomous decision.
From the signature to the end of the study, the investigation team should be accessible and make a follow-up of the consent by monitoring its progress, resolving doubts, informing of any changes, etc. When the study finishes, all the information about the intervention must be noted in his/her medical records. It is advisable to give information about the results of the study to the participants.
During the whole study, feedback will be collected and analysed from patients and research team to assess the process, provide improvements and generate a new starting point for future research, turning informed consent into a dynamic process continually evolving.
Why i-CONSENT?
i-CONSENT places innovation at the centre of its actions:
- Understanding Informed Consent as a communication process
- Including gender perspective in the informed consent process
- Taking into consideration vulnerability factors such as age, gender and cultural background
- Usage of new technologies to better understand informed consent
- Involving all the interested parties in the project
Timeline
The i-CONSENT project main milestones.
Project launch
i-CONSENT starts.
In depth understanding of the context
and workshop with patients to assess needs.
Report on age and gender
related issues regarding Informed Consent.
Digital technologies:
systematic review of the use of digital technologies for Informed Consent.
Development
of innovative tools strategies for patient involvement in clinical trials.
Evaluation
of the draft guidelines and workshops with stakeholders.
Validation
of the guidelines.
Publication
and dissemination of i-CONSENT guidelines for informed consent.