News and Events
Project guidelines now available!
The i-CONSENT Project publishes its final product: "Guidelines for tailoring the informed consent process in clinical studies" Fragment of the guidelines. The i-CONSENT Guidelines are now ready and available for academia, clinical investigators, pharmaceutical...
Biomedical research involving health data in the context pandemic: implications for informed consent
LUMSA University organised Monday, March 22st 2021 the last meeting of its series of encounters to discuss informed consent in pandemic situations. This one, focused on ethical and legal requirements for biomedical research involving health data in the context of...
i-CONSENT presents its guidelines to improve informed consent in clinical studies
The project celebrated its final event: Towards the future of informed consent in clinical research. Yesterday, the i-CONSENT team finally presented its guidelines to improve informed consent in clinical studies. After 4 years of intense work and study in the...
Biological samples and COVID-19: challenges for informed consent
Our partner LUMSA University organised Monday, March 1st 2021 a meeting to further discuss the different emerging ethical problems and implications that COVID-19 has for informed consent. This time, the team invited Prof. M. Toraldo Di Francia to discuss the...
Digital tools in informed consent: a systematic review
New project publication featured in BMC Medical Ethics journal Providing understandable information to patients is necessary to achieve the aims of the Informed Consent process. In recent decades, new, primarily digital technologies have been used to apply and test...
Final Event: Towards the future of informed consent in clinical research
The i-CONSENT project is coming to an end. After 4 years of intense work and study in the field of clinical research and bioethics we are proud to inform you that the project guidelines are ready for publication. These guidelines are the result of profound...
Research protocols and Covid-19: elements for adequate patient information
Following the first session in November, project partner LUMSA University, deemed necessary, given the high level conversation and fruitful discussions, the organization of an additional meeting with expert Dr. Antonio Addis. In this second meeting, Dr. Addis started...
Improving Informed Consent for Novel Vaccine Research in Pediatrics
New project article reflecting on informed consent for vaccine research in pediatric population published in Frontiers in Pediatrics Journal Project partners OPBG, LUMSA and AND CG have published a new article today. The paper is called "Improving Informed Consent for...
5 best popular i-CONSENT posts in 2020
How will the world remember this 2020? For sure, COVID-19 will be on the top 5 most remembered topics, as the pandemic has filled front pages and monopolized conversations for months. What about i-CONSENT? What has interested our readers the most? Find a compilation...
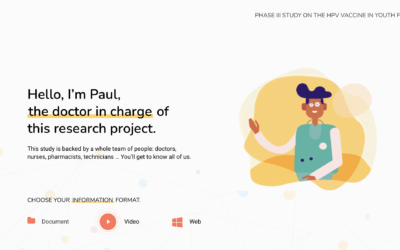
How would the i-CONSENT-based communication materials look like?
i-CONSENT has elaborated examples of communication materials to be used in clinical trials following the project recommendations. The idea was to elaborate a series of materials to deliver all the...
Ethics Committee gives green light to using an i-CONSENT informed consent
The first i-CONSENT informed consent is now circulating. A real Spanish clinical trial has used the project guidelines to elaborate a series of communication materials to provide the information on the study to the participants. The ethics committee has not only...
Project recommendations undergo a final round of revision with experts
The FISABIO team sat together with experts in the field and discussed the guidelines' content using the RAND/UCLA methodology In addition to the validation of the materials elaborated following the i-CONSENT guidelines, it has deemed necessary to evaluate...
Implications of COVID-19 for informed consent
Summary of the sessions organized by our partner LUMSA University Following the sessions organised to discuss informed consent for clinical trials in the context of an emergency setting, find a summary of each meetings' main ideas. Research protocols and COVID-19:...
Conversations on informed consent in the context of the COVID-19
i-CONSENT organizes a series of workshops to discuss informed consent for clinical trials in the context of an emergency setting i-CONSENT partner, LUMSA University, has organized a series of internal online workshops to discuss informed consent in the...
Informed consent in the pandemic context: between bioethics and biolaw
Project publishes an article describing informed consent in biomedical research in the pandemic context in the Journal of BioLaw: Rivista di Biodiritto Clinical research is world widely speeding in order to identify as soon as possible COVID-19 treatment and vaccine....
Fake News in the time of COVID-19: objectives and detection
i-CONSENT has updated its "clues to identify fake news" with a special focus on the COVID-19 pandemic As the pandemic continues to create uncertainty, there is an urgent need for stronger action to manage the wild spread of fake news. To contribute to the cause, we...
i-CONSENT answers the public for Health Literacy Month
The project has made a selection of the most frequently asked questions in internet related to informed consent for Health Literacy Month October is Health Literacy Month. To celebrate it, i-CONSENT has decided to explore people’s questions...
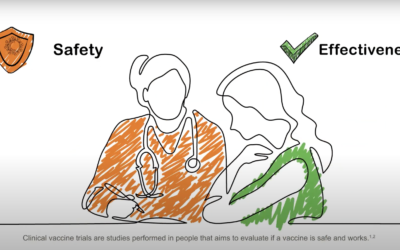
New project product: Clinical trials for vaccines by iCONSENT
The video describes the development of clinical vaccine trials and explains all you need to know before choosing to participate in one The video, elaborated by project partner GSK, explains vaccines' lifecycle from its production in the laboratory to its...
i-CONSENT presents project results on the ESPID virtual meeting
The team is presenting the results on the design thinking sessions organised in the framework of the project i-CONSENT is present on this years’ European Society for Paediatric Infectious Diseases (ESPID) Annual Meeting, which is held virtually 26-29 October...
i-CONSENT validation kicks-off in Spain
The team will evaluate the information materials created following the project guidelines The project validation starts today in Spain and will be followed by a validation in UK and Romania. This validation allows to present the materials elaborated following the...
“Is the digitalisation of clinical research questioning the effectiveness of Informed Consent?”
i-CONSENT publishes a report with the conclusions extracted from its virtual workshop “Rethinking informed consent: emerging challenges in the digital era” The document provides an overview of the online workshop organized by AND-CG and held on 26 March 2020. The...
How to tailor health information to our audience
Strategies and tools to know societies’ concerns and address them in our health messaging, especially in the current COVID-19 emergency Adapting information to the audience is crucial to meet their needs and values. These are unique and influenced by multiple factors...
i-CONSENT Project extends to 2021
The European Commission has granted the i-CONSENT project with a 1-year extension to continue improving informed consent processes. The extension responds to a late modification to widen the project activities and the mitigation of the impact of COVID-19. i-CONSENT...
i-CONSENT virtual workshop: “Rethinking informed consent: Emerging challenges in the digital era”
Find a compilation of all the workshop panels here: Informed consent in the digital era: Is technology a silver bullet? Ethics and data protection considerations https://youtu.be/-_xHjkU107Y Session 1 - Perspectives on the future of consent in clinical research...
Communicating for society: tool for health professionals
Developing easy-to-understand contents to better communicate with patients and citizens in general in the context of the COVID-19 emergency crisis When trying to explain something of our area of expertise to someone, we often get too technical. This is because we...
i-CONSENT COVID-19 glossary of terms
i-CONSENT Project brings science and health closer to society by improving the communication between citizens and professionals. Initially, the Project was designed to make informed consent in clinical research more comprehensible to the participant. However, COVID-19...
Stimulating experts’ views on the challenges of digitalisation and policy developments in clinical research
This virtual workshop organised by i-CONSENT partner AND-CG offered the opportunity to explore different dimensions of informed consent-related practices Around 15 experts gathered yesterday morning for the i-CONSENT webinar "Rethinking informed consent: Emerging...
i-CONSENT organises a multi-stakeholder workshop to discuss ICT in informed consent
The workshop will take place online on the 26th of March The main goal is stimulating dialogue around the challenges presented for consent-related practices in the context of clinical research. The workshop will explore these challenges under the lens of technological...
Women and Girls in Science International Day 2020
As every year, i-CONSENT joins the celebration of Women and Girls in Science International Day. Not only as a matter of solidarity but also as a way of defending social justice in the pursuit of gender equality. In 2020 the i-CONSENT project is reaching its final...
Next i-CONSENT Guidelines Review Meeting
Professor Federico de Montalvo Jääskeläinen will review the project product from an ethical and legal point of view After the fruitful first Guidelines Review Meeting with Prof. Carlos M. Romeo Casabona, for i-CONSENT project it is time to take another step. On...
i-CONSENT presents an informed consent monograph in Rome
Presentation of BioLaw Journal - Special Issue on Informed Consent The past July 2019, the i-CONSENT team published a monograph on informed consent in the BioLaw Journal- Rivista di Biodiritto. Months later, on March 30th, 2020, project members will present the...
iCONSENT participates in a workshop to assess new ways of bringing science closer to society
The event took place in Bonn, Germany, on January 20-21th, 2020 The i-CONSENT team participated last week in a workshop organised by the NewHorrizon project. The event was aimed at exploring strategies to encourage collaboration between investigators and society. The...
The i-CONSENT’s top 5 must-read news stories of 2019
Which of our news articles have catch the eyes of physicians, industry, and medical students more this year 2019? Find a compilation of our most popular posts! 1. i-CONSENT celebrates Women and Girl in Science Internacional Day The winner is this post celebrating the...
i-CONSENT Guidelines start an audit process
The first meeting will be with Prof. Carlos Maria Romeo Casabona, expert in law and bioethics With the product nearly ready, it is time to give the guidelines a final revision. Experts from different fields such as law, bioethics, TIC, gender will be invited to...
What do pregnant women have to say about clinical studies?
New Design Thinking session with pregnant women to incorporate their input to the project's perspective Following our sessions with kids, i-CONSENT met a group of volunteer pregnant women the past November 12th. The idea was to capture their views on informed consent...
F2F Meeting in Rome: Finalising the guidelines
The i-CONSENT Consortium met in Rome to give the final touches to the project's guidelines Consortium representatives sat the past November 4-6 around the table to give the final push the project's product. The i-CONSENT guidelines, with an innovative format, are...
i-CONSENT at the 25th Congress of Spanish Association of Bioethics
i-CONSENT participated the past October 25-26th in the 25th National Congress of the Spanish Association of Bioethics and Medical Ethics (AEBI ) held in Valencia. Topics such as human dignity, the ethics of the end of life, the ethics in assisted reproduction were...
i-CONSENT with Health Literacy Month 2019
6 reasons to participate in clinical trials October is #HealthLiteracyMonth and #iCONSENT is joining the celebration! For this purpose we explored 6 reasons why someone should participate in a #clinicaltrial. Before a treatment is commercialized it must undergo a...
i-CONSENT at the X Congress of Spanish Biobanks
Our technical coordinator explained the i-CONSENT project and its applications to biobanks More than 200 representatives from Spanish biobanks met the past october 17 and 18, 2019 in valencia. The experts discussed the challenges and the future of biobanks. In this...
i-CONSENT’s second design thinking session with children
After the first meeting with our youngest experts, i-CONSENT took a step forward and helped children design an informed consent “prototype” by themselves. Last week a total of 6 children aged 12 and 13 participated in the second edition of the i-CONSENT Design...
i-CONSENT joins again European Researchers’ Night 2019
Engaging students through roll plays on the topic of informed consent The i-CONSENT team held interactive sessions for 16-18 years old students about informed consent at LUMSA University for the European Researchers’ Night 2019. More than 70 students had the...
New session with kids to include their perspective in the project’s guidelines
Design Thinking Methodology will be used again to connect with a group of children and learn their opinion October the 3rd has been the chosen date to perform another Design Thinking session with children. The same group that was gathered in June will now take a step...
“We want to identify the main needs of patients in specific situations in order to overcome them”
Interview with Laura Palazzani, i-CONSENT Coordinator for Lumsa University Laura Palazzani is Full Professor of Philosophy of Law, Biolaw and Bioethics at LUMSA University of Roma. She is Vice-President of the Italian Committee for Bioethics (since 2007) at the...
New concept, new guidelines: conclusions after the meetings
i-CONSENT develops a route map for professionals involved in the design of informed consent process After three days of intense work, i-CONSENT can finally say that the process of elaboration of the guidelines is coming to an end. The project team flipped around the...
F2F Meeting in Valencia: A step closer of our final product!
From today until Friday, Consortium members coming from UK, Italy and Spain will meet up again to discuss the draft version of the project's guidelines to improve informed consent. The meeting will take place in Valencia, at Fisabio's headquarters, and will focus on...
Why is i-CONSENT so relevant? GSK answers
Interview with GSK representatives about the importance of the project's research https://www.youtube.com/watch?v=i03rvV12vrs
Interview with Jaime Fons-Martínez, i-CONSENT Technical Coordinador
He explains the current project developments and the methodology used to meet its objectives Jaime holds a Degree in Sociology and Business Management and Administration, Master’s Degree in Sociology and Anthropology. Moreover, He has been working as sociologist...
i-CONSENT present at the European Research Night 2019
i-CONSENT will participate in this year's edition to introduce the subject of informed consent in clinical studies The LUMSA University participates for the fourth consecutive year in the European Researchers' Night to bring researchers and their work close to...
When assent becomes as important as consent
Minor’s Assent in Medical Research: Differences between the Scientific Literature and the Legal Requirements Informed consent in medical studies with minors is a subject of great importance. There is still a debate about the requirements to consider this assent valid...
iCONSENT at the X Spanish Congress of Biobanks
The event will take place in Valencia on October 17-18th i-CONSENT will be present at the X Spanish Congress of Biobanks on October 17-18th (2019) in Valencia. Jaime Fons (Fisabio) will represent the project and will intervene regarding the project's developements,...
Has paper-based Informed Consent era come to an end?
eConsent is consolidating itself as benchmark in the development of health-related studies Although there has been a change since those everlasting 15-20 page long Informed Consent (IC) documents, when participating in clinical studies potential participants still...
5 frequently asked questions (FAQs) on Informed Consent
Many doubts arise when taking part on a clinical study. More than a mere disclaimer, informed consent processes exist to tackle them. Based on frequent Google searches, i-CONSENT has selected 5 frequently asked questions on Informed Consent that you might have come...
i-CONSENT involves children in the project through Design Thinking
i-CONSENT team organised last week a workshop with children to assess their knowledge on clinical studies and develop strategies to better communicate with them Participation of children in clinical studies is essential but still minoritary. How to communicate with...
Gaps and challenges to the Informed Consent Process by i-CONSENT
It is generally agreed that informed consent is a process whose effectiveness is a factor of a few core criteria. These criteria are typically held to include concepts such as disclosure, understanding, capacity, and voluntariness. Though guidelines differ somewhat in...
i-CONSENT brings project conclusions before the Spanish Research Ethic Committees
The VI ANCEI Congress was celebrated on May 30 and 31 in Tarragona For second year in a row, the i-CONSENT project participated in the ANCEI Congress in its VI edition, taking the first project results before the Spanish Research Ethic Committees. i-CONSENT...
Next steps for i-CONSENT
From WP2 and WP3 to the elaboration of the guidelines With approximately one year of project left, i-CONSENT is starting to translate the findings into a set of guidelines to improve Informed Consent. The conclusions of i-CONSENT WP1 (Work package 1, A...
Health fake news and how to fight them by i-CONSENT
Fake news have currently become one of society's major concerns, the harm they produce in several fields as politics or health, or their impact in individual and collective memories, has placed them on the spotlight. Society’s perception of their influence and...
Inclusion of research participants from different cultural and religious backgrounds in clinical trials
Development of new strategies through “Design Thinking Exercise” methodology What are the most frequent doubts and concerns of research participants when they are offered the opportunity to enrol in a clinical study? How can we facilitate communication between...
i-CONSENT celebrates Women and Girl in Science Internacional Day
Science and gender equality are both vital for development, yet today full and equal access to research is a privilege of the few. This problem affects women and girls to a greater extent who have been traditionally pushed away from certain fields. Women and...
Workshop: A step closer to improving Informed Consent
i-CONSENT presents the draft guidelines to main stakeholders in Brussels i-CONSENT has reached the second stage of the project. The draft guidelines for improving the Informed Consent Process are now finished and next step is its revision. The recommendations...
Workshop: i-CONSENT meets up with International Bioethics Committee Members
i-CONSENT Consortium will gather at Università LUMSA to discuss main findings of the project and comments elaborated by International Bioethics Committee members. The Consortium will also identify any gap in i-CONSENT work and suggest actions to improve strategies...
i-CONSENT enables collaboration among cultural mediators to evaluate new design methodology
LUMSA University meets with cultural mediators in Rome to test Design Thinking LUMSA University has organizanised in conjunction with OPBG a series of encounters with cultural mediators to test a new design methodology: Design Thinking. Last Wednesday was the...
‘Social’ informed consent or the end of the gap between adolescents and clinical trials
A Workshop with several group of students (age 16-18) reveals that adolescents would rather receive informed consent through social media and audiovisual material than in a traditional way. Social media appears to be the most popular solution to improve informed...
i-CONSENT presents first results in Rome
“Ethical and Legal Issues concerning Informed Consent”, seminar in Rome LUMSA team presented last Monday first i-CONSENT results within the seminar “Ethical and Legal Issues concerning Informed Consent” held in Rome. The...
Informed Consent: What are the ethical challenges at stake?
Clinical trials are key to ensuring scientific progress in the medical field and gaining breakthrough knowledge on safety and effectiveness of drugs and therapies, but they entail risks for enrolled participants, as one deals with non-validated interventions....
Clinical Research i-CONSENT A to Z
Health literacy should be more than just understanding health care terminology, but it is a good starting point. i-CONSENT has developed a Clinical Research A to Z on the occasion of the #HealthLiteracyMonth celebrated in October. Accompany us through some of the...
Exploring i-CONSENT within the European Researchers’ Night
The European Researchers' Night, is around the corner (28th September 2018) and i-CONSENT research project doesn’t want to miss the date. Our consortium member LUMSA University, participating for third consecutive year, will open its offices in Rome and Palermo to...
“Multiculturalism and Interreligious Perspective on Informed Consent” Workshop
The UNESCO Chair in Bioethics and Human Rights hold its 6th international Bioethics, multiculturalism and religion workshop to discuss issues of informed consent and clinical research on February 21-23. As part of the i-Consent consortium (a project funded by the...
Adequacy, coherence and cohesion in Informed Consent
Minors are traditionally left out of bureaucracy for protection reasons until they trespass the threshold of adulthood set by the law. Consequently, adults must provide decisions in their place taking into account their best interest. Even so, the increasing amount of...
Specific communication strategies for children essential in IC
For many years, procedures, medicines and tests performed in children, have not been studied or authorized specifically for them. The dynamic was based in transferring the results obtained from adult population to paediatric population. The same happened with Informed...
Press release Health literacy month
I-Consent partners in Rome, a meeting to remember !
The first I-Consent consortium meeting took place in Rome from december 4 to 5. All the stakeholders and contributors involved in the project attended the event hosted by Lumsa University and OPBG. This meeting aimed to present the progress made in every work package...
COVID-19 contact tracing apps: ethical issues and implications for informed consent
Summary of the i-CONSENT’s conversations on informed consent in the context of the COVID-19 last session The last intervention took place December 15th and was conducted by Prof. Assuntina Morresi. It revolved around the ethical issues and implications for informed...
Research Ethics and Ethics of Treatments for COVID-19
Summary of the i-CONSENT’s conversations on informed consent in the context of the COVID-19 fourth session Prof. C. Caporale started off by pointing out the two stage phase for the ethical approval of clinical trials in Covid-19 times in Italy. The first...
Clinical research on COVID-19: bioethical aspects
Summary of the i-CONSENT's conversations on informed consent in the context of the COVID-19 second session During his lecture on Monday, November 23rd 2020, Prof. d’Avack focused on informed consent in the context of trials currently in progress in the therapeutic...
Research protocols and COVID-19: adequate patient information
Summary of the first session of the i-CONSENT's conversations on informed consent in the context of the COVID-19 In his lecture on Monday, November 16th 2020, Dott. A. Addis stressed the importance of conducting a sound and robust scientific research, to counteract...
Privacy and personal data after COVID-19: implications for informed consent
Summary of the i-CONSENT’s conversations on informed consent in the context of the COVID-19 third session During his talk on December 9, 2020, Prof. G. Comandé, put forward several important arguments regarding COVID-19 implications for informed consent....
#healthliteracymonth in a nutshell
Health Literacy Month is now over, and we are very proud to have participated to the worldwide awareness-raising event through our Twitter campaign. Still wondering what is Informed Consent? These tips are for you! Feel free to share and/or retweet our posts with...
I-Consent taking part in #healthliteracymonth campaign
Health Literacy Month is an annual worldwide awareness-raising event that has been created in 1999 by Helen Osborne, founder of Health Literacy Consulting. Helen is a health literacy consultant who helps professionals communicate health information in ways that...
The power of patients in clinical trials
Today, September 7th 2017, is the #PatientsHavePower day! This initiative is organized by Clara, an organization that gives patients the opportunity to join and participate in clinical trials, in order for them to have access to the newest treatments, related to their...
New EU project aims to improve guidelines for Informed Consent
The informed consent (IC) process allows the subject to voluntarily decide whether or not his/her participation in research. Therefore, autonomy of the patient in the decision of having a medical procedure or participating in clinical research is of major importance....